ANSI/AAMI ST108: Its Impact on Sterile Processing Water Quality

What Is ST108
ANSI/AAMI ST108:2023, Water for the Processing of Medical Devices (ST108), is the most recent enforceable standard guiding how water should be treated and managed in sterile processing settings.(1) This standard was developed by the Association for the Advancement of Medical Instrumentation (AAMI) alongside the American National Standards Institute (ANSI) and replaces the previous AAMI TIR34 guidance.(2)
Unlike TIR34, a Technical Information Report and non-mandatory, ST108 is a formal standard enforceable by regulatory and accreditation bodies like The Joint Commission (TJC) and the Centers for Medicare & Medicaid Services (CMS).(3) ST108 expands the scope of application to include measurable water-quality requirements and a detailed framework for water quality throughout every stage of medical device reprocessing.
Why Water Quality Is Important in Sterile Processing
Sterile Processing Departments (SPDs) play a critical role in cleaning, disinfecting, sterilizing, and preparing medical instruments and devices for reuse.(4) Throughout this cycle, the quality of water directly affects patient safety and device functionality.
ST108 categorizes water into three types:
- Utility Water – Municipal or supply water used generally for flushing or rinsing
- Critical Water – Water treated to eliminate microorganisms along with organic/inorganic contaminants; used for final rinses and high-level disinfection(1)
- Steam – Vaporized water used in steam sterilizers, which needs to meet specific purity standards to prevent device contamination(5)
Patient and facility safety is heavily reliant upon water quality. In sterile processing specifically, poor water quality introduces a number of risks including:
- Increased potential for infection (e.g., surgical site infections)
- Pyrogenic reactions due to endotoxin exposure(3)
- Pitting and corrosion of devices, which in turn creates crevices for tissue and contaminants to collect
- Device malfunction
- Ineffective cleaning or disinfection due to biofilm buildup and improper rinsing(6)
- Failure to meet reprocessing criteria under Spaulding Classification, especially for semi-critical and critical devices(7)
How Water Quality Is Measured in ST108
Included in ST108 are defined sets of testing categories, frequencies, and sample points, as well as quality standards for each of the three water types defined above. The testing categories (listed below) define what data must be collected and then measured according to the ST108 quality standards.
Testing Categories:
- Total Hardness
- Conductivity
- pH
- Total Alkalinity
- Bacteria
- Endotoxins
- Ionic Contaminants
- Total Organic Carbon (TOC)(8)
Of particular importance to patient safety are the quantitative limits defined for the presence of bacteria (CFU/mL) and endotoxins (EU/mL) within Critical Water, due to the risk of patient harm. The required applications of Critical Water throughout a sterile processing cycle carry the highest risks of device recontamination and drive the stringent quality oversight defined throughout ST108.(1)
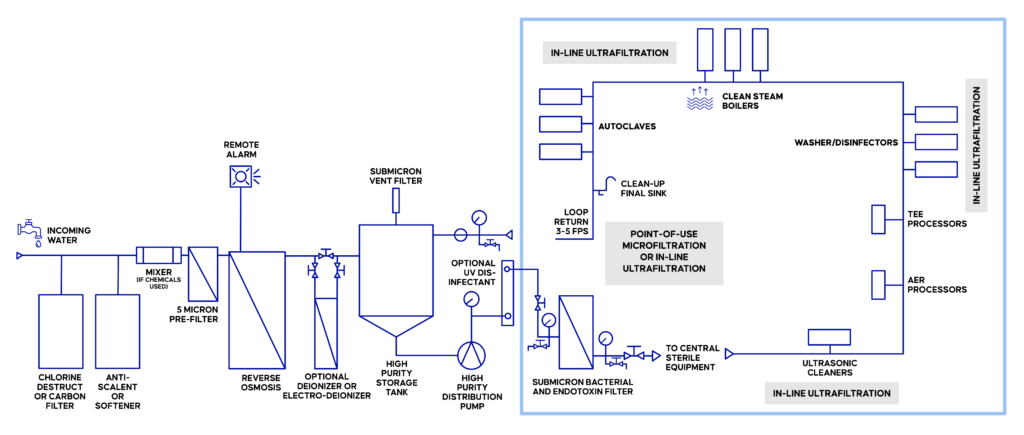
Treatment Technologies and Equipment for Critical Water Quality Compliance
To meet the demanding requirements of ST108, SPDs must use a combination of water treatment technologies, taking into consideration the strengths and weaknesses of each option. A multi-tiered approach ensures comprehensive purification, especially important with Critical Water creation and use.
Examples of Critical Water Treatment and Equipment(1)
Reverse Osmosis (RO)
Benefits:
- Removes up to 95–97% of inorganic, organic, microbial, and endotoxin contaminants (as per ST108)
- Produces high-purity water that’s suitable for Critical Water applications
- Helps maintain compliance with ST108 bacteria and endotoxin limits when regularly maintained
Shortcomings:
- Doesn’t guarantee complete endotoxin removal—its effectiveness varies based on system conditions
- Can be affected by membrane fouling, necessitating pretreatment and consistent upkeep
- Downstream contamination is possible; best when followed by post-RO, point-of-use filtration
Deionization (DI)
Benefits:
- Eliminates charged ions, lowering conductivity to extremely low levels
- Commonly employed as a secondary polishing step following RO
- Compact and scalable for various SPD configurations
Shortcomings:
- Does not eliminate bacteria, viruses, or endotoxins
- Resin beds can turn into microbial reservoirs if not maintained regularly
- Requires frequent regeneration or cartridge changes
Ultraviolet (UV) Disinfection
Benefits:
- Offers chemical-free disinfection by inactivating bacteria and some viruses
- Leaves no residuals in the water
- Useful in recirculation loops or as an adjunct to RO/DI
Shortcomings:
- Does not deactivate endotoxins
- Lacks a residual effect—only functions at the exposure point
- Requires clear water and well-maintained lamps for effectiveness
Storage Tanks and Distribution Loops
Benefits:
- Deliver a buffered water supply during heavy usage
- Help sustain steady flow, temperature, and pressure
- Assist closed-loop circulation to maintain water quality
Shortcomings:
- Stagnation and biofilm buildup are concerns if recirculation is poor
- Demands regular disinfection and system monitoring
- Can reintroduce contaminants without proper validation
The Importance of Endotoxin Filtration for ST108 Compliance
A key update from ST108 is the increased focus on endotoxins—toxic byproducts from bacterial cell walls that standard disinfection or steam sterilization may not remove.(3)
Various clinical sources emphasize that while steam effectively kills bacteria, it doesn’t neutralize endotoxins. In fact, thermal sterilization can break down gram-negative bacteria, releasing lipopolysaccharides (LPS) and heightening the endotoxin load. These fragments are heat-stable and can remain biologically active even after high temperatures, posing risks to patients—especially with invasive or implantable devices.(9)
Given this, facilities adhering to ST108 need to prevent endotoxin contamination well ahead of sterilization. Effective measures include reverse osmosis and membrane filtration.
However, not all membrane filters perform the same. The most common models for endotoxin removal depend on membranes with positive electrostatic charge. This filter type offers a high flow rate, but requires continuous maintenance, and the performance is both inconsistent and influenced by water chemistry.
Other models leverage size exclusion to retain endotoxins—these are far less common due to the extremely small size of endotoxins. Size exclusion filters for endotoxins must use technology with pore sizes of 0.01 or smaller to be effective. Such filters are also known as ultrafilters. They are not only effective at retaining endotoxins, but bacteria as well. The benefits of this filter type include consistent, reliable performance, as well as the reduced maintenance requirements. However, ultrafilters with ultrafiltration-level pore sizes do bring a reduction in flow rates and are typically best utilized directly at points-of-use.(10)
Summary
ANSI/AAMI ST108 raises the bar for all water quality used within sterile processing. By implementing multiple, validated treatment solutions with filtration at points-of-use, facilities can:
- Minimize Contamination risk
- Meet or exceed quantatative limits for bacteria and endotoxins
- Enhance patient safety while maintaining accreditation standards
(1) ANSI/AAMI ST108:2023 – Water for the processing of medical devices
(2) AAMI TIR34:2014/(R)2021 – Water for the reprocessing of medical devices
(3) CDC – Guidelines for Disinfection and Sterilization in Healthcare Facilities (2008, updated 2019)
(4) FDA – Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling Guidance (2015)
(5) AAMI ST79:2022 – Steam sterilization and sterility assurance in health care facilities
(6) Rutala WA, Weber DJ (2016) – CDC Disinfection and Sterilization Guidelines
(7) Spaulding Classification System – Referenced in CDC guidance
(8) USP <1231> – Water for pharmaceutical purposes
(9) Peer-reviewed research on steam and endotoxins: e.g., Favero et al., Journal of Applied Microbiology; Walker et al., American Journal of Infection Control
(10) Nephros, Inc. – Infection control water filtration solutions. www.nephros.com
(11) IAHCSMM Education Resources – Sterile processing technician training material
