Education
Could a COVID vaccine threaten an endangered species and impact human health in the process?
By Kimothy Smith, DVM, PhD
What do you find at the intersection of COVID-19 and endangered species? There is a joke in there somewhere, but if you’re drawing a blank, we’ll fill it in for you: horseshoe crabs.
Yes, believe it or not, these prehistoric, ocean-dwelling creatures may find themselves on the edge of extinction after a COVID-19 vaccine is mass-produced and distributed worldwide. These large, rather ungainly arthropods have been roaming beaches and oceans for over 300 million years, surviving multiple ice ages and even the asteroid impact that likely wiped out the dinosaurs.
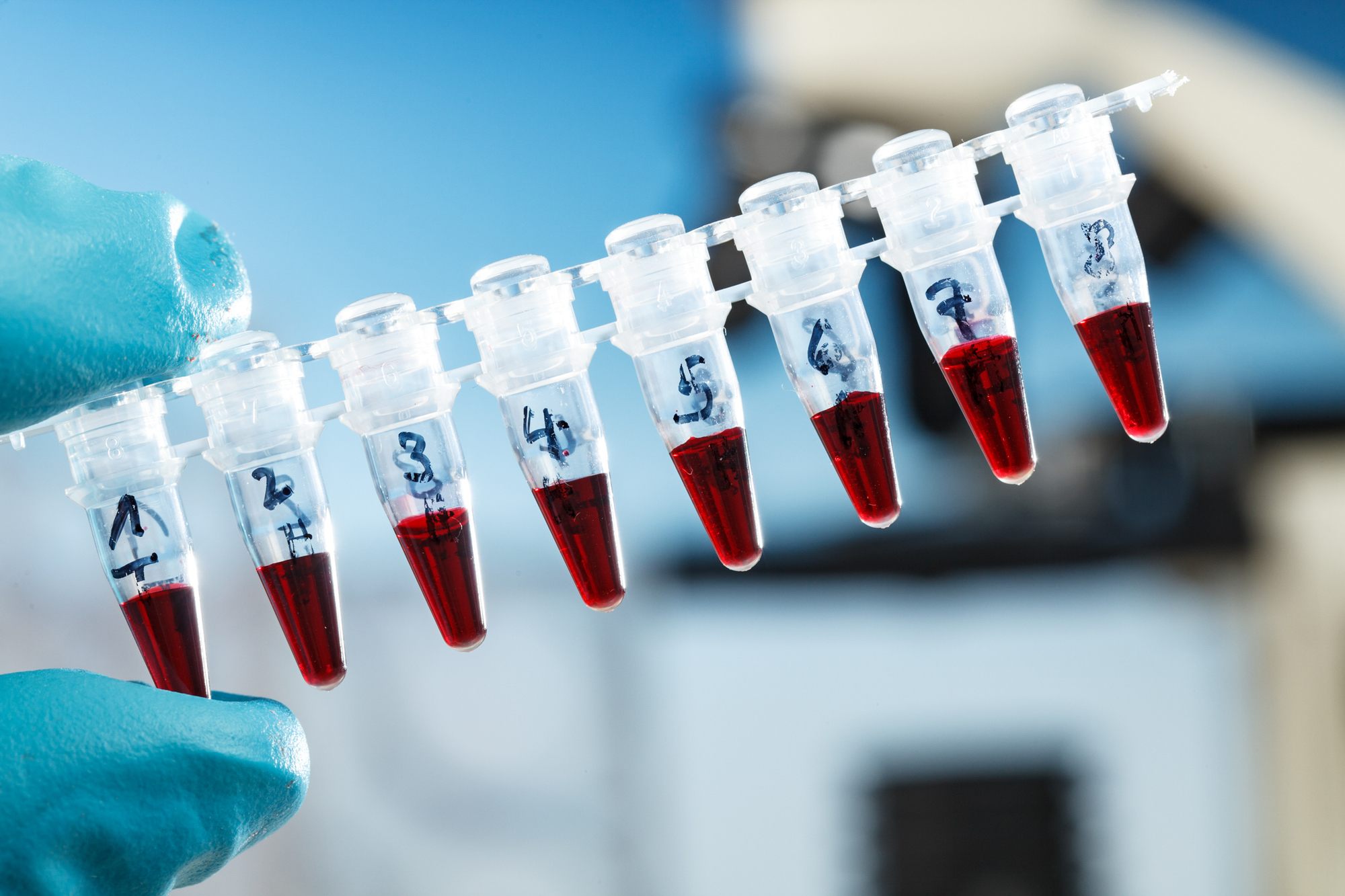
Few people are probably aware, though, that amebocytes (mobile cells found in invertebrates) from these gentle creatures are indispensable to modern medicine. Since its FDA clearance in 1977, the limulus amebocyte lysate (LAL) assay has been the standard test for contamination from Gram-negative bacteria for all medicines, as well as IV drips and implantable medical devices.
Without the crabs’ milky blue blood, modern medicine would simply not exist in its present form and the human lifespan would likely be markedly shorter.
So, every year more than 400,000 horseshoe crabs are collected – many from the US Atlantic coast – and bled en masse to procure the basis of the LAL assay. Doing a Google image search on this topic is not for the faint of heart: some find the bleeding process to be positively Frankenstein-esque.
The companies that conduct the gathering-bleeding process claim that most of the crabs are returned to the wilderness largely unharmed. Some studies have shown, though, that as many as half of them die in the process.
Whatever the true figure, most can agree that a great many horseshoe crabs sacrifice their lives in the process, which brings us to our current impasse: given that billions of COVID-19 vaccine doses will be needed in coming months and years, the worry is that horseshoe crab populations, already under pressure, could be impacted beyond repair, with serious consequences for human health.
Bearing in mind that all prescription drugs, vaccinations, and implantable medical devices are as safe as they are because of the horseshoe crabs, it is in our rational self-interest – and indeed, imperative – that we find a way to avoid destroying them. If the horseshoe crab ceases to exist, or even if their population declines precipitously, modern medicine ceases to exist in its present form. It’s really that simple, which is why more effort and resources need to go into developing alternatives.
That’s why we, at Nephros, are in the final stages of developing an LAL alternative, DialyPath, specifically for use in ensuring the safety of dialysis machines.
LAL testing alternatives
During the last 19 years, there have been efforts to develop alternatives to the traditional LAL test and make endotoxin screening a more sustainable process and reduce impact on horseshoe crab populations. Arguably the most well-known of those efforts, recombinant-Factor C (rFC), was introduced by researchers at the National University of Singapore in 2001 and was accepted by the European Pharmacopoeia as an alternative to the LAL test in 2016. The method uses a synthetic version of the Factor C clotting protein found in horseshoe crab blood, but still requires some horseshoe crab DNA for lab manufacturing. By using the recombinant-Factor C, scientists can achieve similar detection of endotoxin quantities by measuring cleaved fluorescence substrates attached to the protein. Although rFC has demonstrated comparable sensitivity to LAL testing, it has yet to be adopted by the United States Pharmacopeia (USP) or the Food and Drug Administration (FDA). Furthermore, although rFC helps reduce dependency on the horseshoe crab population, it does not wholly eliminate them as a resource for endotoxin screening research.
There is an alternative entirely divorced from a dependence on horseshoe crabs called, Monocyte Activation Test (MAT). Different from LAL testing, MAT uses human blood and the cytokine response in the body’s innate immune system to measure endotoxin presence and quantification. The other added benefit with MAT is it measures all pyrogens present (endotoxin is one type of pyrogen), which includes other microbe particles from viruses, gram-positive bacteria, and yeast. The MAT has been accepted as a compendial method for pyrogenic testing in the European Pharmacopoeia since 2010 but has not been adopted by the USP.
US and international regulators, like the FDA, strictly adhere to the standards for validated alternatives described in the USP and European Pharmacopoeia. Given the rigid standards, some companies have historically been reluctant to submit alternative testing to peer review validation and approval. But, as advancements in molecular biology have occurred, coupled with more affordable technologies, there is now increased willingness and incentives for LAL testing alternatives to be inserted in the market.
Want to know more?
Reach out for quotes, additional product details, inventory status, installation questions and more.